RNA and molecular biology
Research into RNAs (ribonucleic acids) is essential to discovering the causes of MND and developing novel therapeutic strategies including personalised/precision medicine.
RNA and molecular biology
Research into RNAs (ribonucleic acids) is essential to discovering the causes of MND and developing novel therapeutic strategies including personalised/precision medicine.
DNA and RNA
The genetic master plan of every living cell is contained within its DNA (deoxyribonucleic acid) and its subunits, the genes. In order to make use of genetic information, copies are produced in the form of RNAs (ribonucleic acids) in a process called transcription.
RNAs are very diverse molecules and can serve either as an instruction for the translation of the RNA into a protein or as enzymatic, regulatory or structural molecules in their own right (i.e. protein-coding and non-coding RNAs, respectively).
RNA and Motor Neurone Disease
The amounts and sequences of several thousands of RNA molecules are altered in the most common subtypes of MND (C9ORF72, SOD1, TARDBP/TBP-43, FUS). However, the proportion and the identity of those abnormal RNA changes that cause motor nerve injury remains largely unknown. In addition, how the downstream functional effects of the abnormal RNA changes has on making proteins, the cell’s building blocks, is also poorly understood. Most changes are likely to have occurred not as a cause but as a consequence of previous dysregulations. Thus, the challenge is to find which disease-changed amounts of RNAs and proteins trigger MND onset and progression.
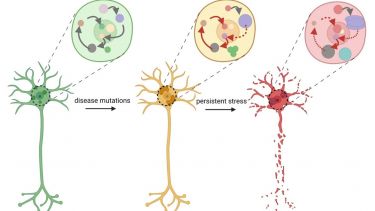
RNA-rich biomolecular condensates and MND
Cells have to coordinate a large number of biochemical reactions. They do so by segregating molecules into organelles many of which form via biological phase separation, like oil separates from water. Resultant biomolecular condensates are usually rich in RNA and act as “microreactors” and accurate sensors of changes in the cell environment thereby helping cells to cope with stress. Their homeostasis is especially important in large, long-living cells neurons. In neurodegeneration, e.g. MND, components of biocondensates are affected by mutations and are found within pathological protein aggregates. As a result, the entire biocondensate network collapses.
Our research
We identify all disease-changed RNA molecules using next generation RNA sequencing in transcriptomics studies and a novel translatome approach, which allow measuring changes in RNA molecules associated with ribosomes, the protein factories. Our aim is to identify all abnormal RNA molecules that cause MND in order to provide new gene therapy, drug and peptide targets for the development of novel therapeutic strategies for neuroprotection. We use a variety of biochemistry, molecular and cellular biology technologies in cell and animal models (Drosophila, mice) as well as in patient-derived motor neurons and post-mortem human brains to carry out these investigations. We also use in vitro systems, purifying recombinant proteins and radioactively-labelling RNAs for the reconstitution of protein: RNA complexes which reveal detailed molecular and structural mechanisms causing RNA defects.
Modelling disease-associated phase separation
We use multiple tools to model, research and therapeutically modulate abnormal phase-separated structures in MND, both in cells and in the test tube. For example, we use plant proteins (e.g. Cry2) that, when attached to disease-linked RNA-binding proteins, cause their phase separation in human cells after stimulation with blue light. This high-controllable system that does not require chemicals allows us to induce phase separation and pathological aggregation on-demand and study their effects on neuronal homeostasis.
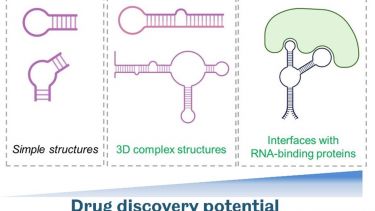
Therapeutic modulation of RNA with small molecule drugs
Traditional, protein-focused small molecule drug discovery poorly covers the protein components of biomolecular condensates that are largely ‘unstructured’. Recently RNA targeting by small molecules has gained more traction, and we are using this state-of-the art approach for therapeutic targeting of RNA-rich phase separated structures in neurodegeneration. RNA-centric approach allows therapeutic modulation of both protein-coding and non-coding RNA thereby greatly expanding the “druggable genome”.
Developing gene therapy medicinal products
The identification of disease-changed RNA and protein amounts or abnormal RNA:protein contact is restored to normal physiological levels using gene therapy approaches, which allow engineering the genetic make-up of harmless adeno-associated virus to increase or reduce the amounts of disease-changed RNAs of interest involved in the neurodegenerative process. The therapeutic efficacy and safety of these virus is further tested via injection into the cerebrospinal fluid of mouse models, prior to taking the most promising academic discoveries made in the lab in further development on the translational path for clinical trials.